Leading maritime community ! [email protected]
Strictly speaking, a perfect gas is an ideal which can never be realized
in practice. The behaviour of many “real” gases is very similar to the behaviour of a perfect gas.
Two of the laws describing the behaviour of perfect gases are Boyle’s Law
and Charles’ Law.
Table of Contents
ToggleThis law may be stated as follows:
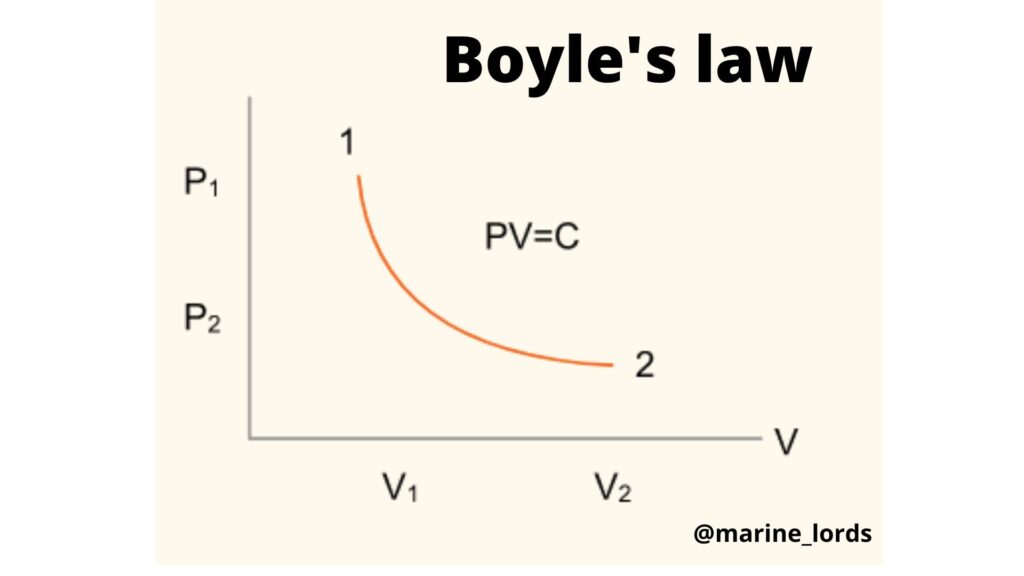
Provided the temperature T of a perfect gas remains constant, then the volume V of a given gas is inversely proportional to the pressure P of the gas,
i.e. P x V = constant if the temperature remains constant.
If a gas changes from a state 1 to a state 2 during a constant
temperature process (isothermal), then
P 1 x V 1 = P 2 x V 2 = A constant
If the process is represented on a graph having axes of pressure P and volume
V, the result will be as shown in the graph below.
The curve is known as a rectangular hyperbola, having the mathematical equation.
xy= constant
Provided the pressure P of a given mass of gas remains constant, then the volume V of the gas will be directly proportional to the absolute temperature T of the gas,
i.e.
V = constant x T
V/T = constant for constant pressure P.
If a gas changes from state 1 to state 2 during a constant pressure process, then
V1 /T1 = V2 /T2 = constant
If the process is represented on a P-V diagram, the result will be as
shown in the figure below
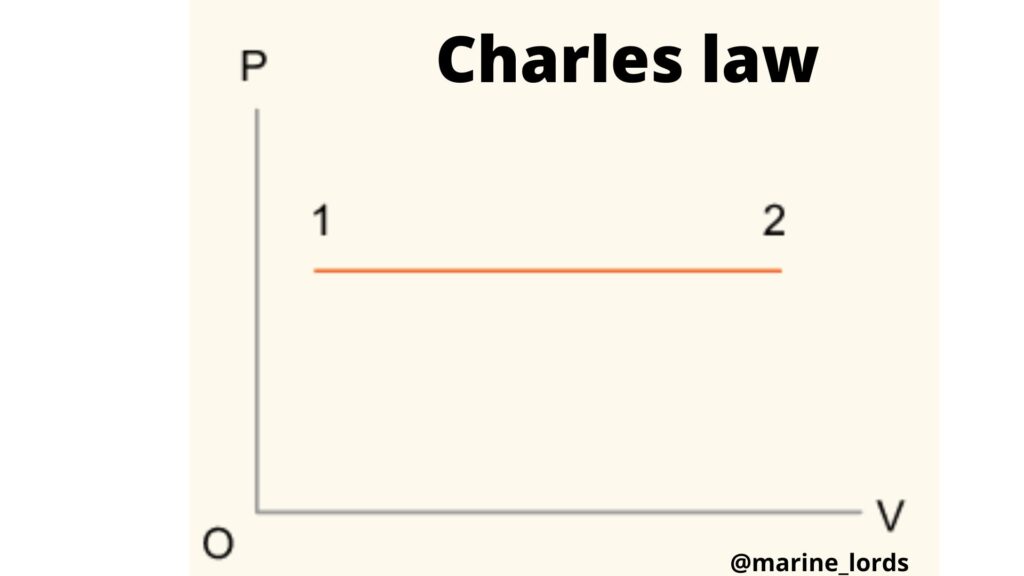
The pressure, volume and temperature of a gas may all change at once
from P1V1 and T1 to P2V2 and T2.
In this case, because pressure changes, Charles’ Law will not apply and because the temperature changes, Boyle’s Law will also not apply. This change of state may therefore be regarded as taking place in two
stage:
a) By a change according to Boyle’s Law
b) A change according to Charles’ Law
By doing this it will be found that the following will apply.
(P1V1/T1 )= (P2V2/T2)
This result may be expressed thus:
The product of the pressure and the volume of a quantity of gas divided by its absolute temperature is a constant and this may be written as
PV/T = C or PV = CT where C is constant.